Overview¶
Aim¶
To interpret the simulation results, it is essential to compare the direction of the perturbation effect with the developmental flow of the trajectory. This comparison will allow us to assess the perturbation’s overall effect (i.e. block or promote differentiation), and intuitively understand how TF is involved in cell fate determination during development.
Method summary¶
For that purpose, we will introduce how to calculate the direction of differentiation using “pseudotime estimation” and “gradient calculation”. Here’s an overview of how to do this:
Calculate the pseudotime using the diffusion pseudotime method (dpt).
Transfer pseudotime data to grid points
Calculate the 2D gradient vector field using the pseudotime on the grid points
Compare the perturbation and pseudotime vector fields by computing their inner product values.
In this notebook, we will do step1: pseudotime calculation. This step is divided into the following parts:
Set the lineage information and split the cells into several lineage brahches.
Set root cells for each lineage.
Calculate pseudotime with DPT algorithm.
Re-aggregate scRNA-seq data into one data
Custom class / object¶
Pseudotime_calculator: This is a class for the pseudotime calculation. This class help us calculate pseudotime from scRNA-seq data. We need to specify a root cell. Also, scRNA-seq need to have a diffusion map. >Under the hood, the Pseudotime_calculator uses “dpt” algorithm. For more information of dpt algorithm and root cell, please look at the scanpy web documentation. https://scanpy.readthedocs.io/en/stable/api/scanpy.tl.dpt.html#scanpy.tl.dpt
Data¶
Pseudo-time calculation requires preprocessed scRNA-seq data in anndata format. You need to do neighbor calculation and diffusion map calculation in advance. If you have processed the scRNA-seq data according to our tutorial, these calculations have already been performed. - Neighbor calculation: https://scanpy.readthedocs.io/en/stable/generated/scanpy.pp.neighbors.html#scanpy.pp.neighbors - Diffusion map calculation: https://scanpy.readthedocs.io/en/stable/generated/scanpy.tl.diffmap.html#scanpy.tl.diffmap
Install additional python package (This is an optional step.)¶
This notebook we recommend using another python package, plotly.
Please install plotly in advance.
pip install plotly
Plotly is a toolkit for interactive visualization. We recommend using plotly to pick up root cells in this notebook. For more information, please look at plotly web site. https://plotly.com
Caution¶
Here, we will introduce an example of a pseudotime calculation using the diffusion pseudotime method. This is NOT CellOracle analysis itself, but just a data preparation step. In addition to the dpt method introduced here, CellOracle also accepts output from any pseudotime method.
0. Import libraries¶
0.1. Import public libraries¶
[1]:
import copy
import glob
import time
import os
import shutil
import sys
import matplotlib.pyplot as plt
import numpy as np
import pandas as pd
import scanpy as sc
import seaborn as sns
from tqdm.auto import tqdm
#import time
0.2. Import our library¶
[2]:
import celloracle as co
from celloracle.applications import Pseudotime_calculator
co.__version__
[2]:
'0.10.1'
0.3. Plotting parameter setting¶
[3]:
#plt.rcParams["font.family"] = "arial"
plt.rcParams["figure.figsize"] = [5,5]
%config InlineBackend.figure_format = 'retina'
plt.rcParams["savefig.dpi"] = 300
%matplotlib inline
1. Load data¶
If you have
Oracleobject, please run 1.1.[Option1] Load oracle data.If you have not made an
Oracleobject yet and want to calculate pseudotime usingAnndataobject, please run 1.2.[Option2] Load anndata.
In this notebook, we will load demo Oracle object and add pseudotime information to it.
1.1. [Option1] Load oracle data¶
[4]:
# Load demo scRNA-seq data.
oracle = co.data.load_tutorial_oracle_object()
# Instantiate pseudotime object using oracle object.
pt = Pseudotime_calculator(oracle_object=oracle)
1.2. [Option2] Load anndata¶
[5]:
# Load demo scRNA-seq data.
adata = co.data.load_Paul2015_data()
# Instantiate pseudotime object using anndata object.
pt = Pseudotime_calculator(adata=adata,
obsm_key="X_draw_graph_fa", # Dimensional reduction data name
cluster_column_name="louvain_annot" # Clustering data name
)
2. Pseudotime calculation¶
2.1. Add lineage information¶
Pseudotime calculation can be done for each lineage. We can define these lineages below.
2.1.1 Check clustering unit¶
[6]:
print("Clustering name: ", pt.cluster_column_name)
print("Cluster list", pt.cluster_list)
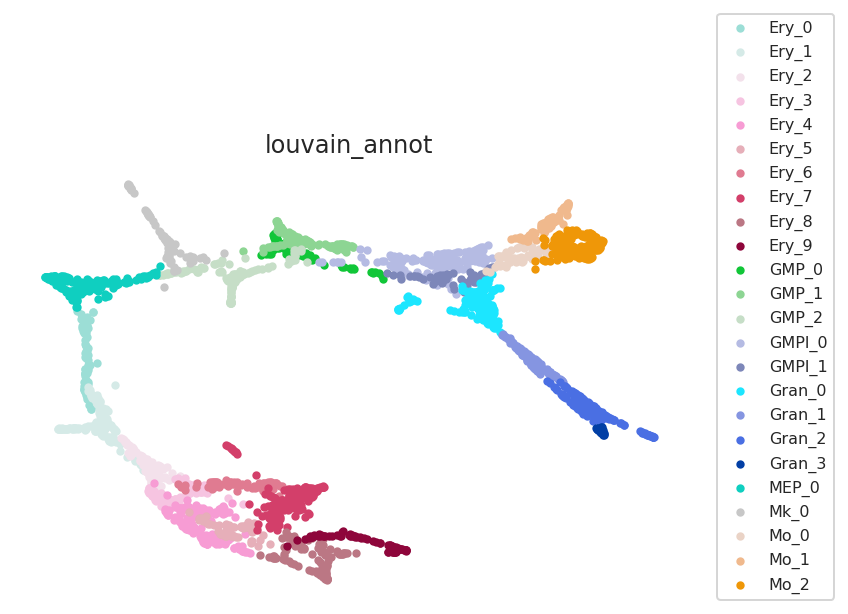
Clustering name: louvain_annot
Cluster list ['Ery_0', 'Ery_1', 'Ery_2', 'Ery_3', 'Ery_4', 'Ery_5', 'Ery_6', 'Ery_7', 'Ery_8', 'Ery_9', 'GMP_0', 'GMP_1', 'GMP_2', 'GMPl_0', 'GMPl_1', 'Gran_0', 'Gran_1', 'Gran_2', 'Gran_3', 'MEP_0', 'Mk_0', 'Mo_0', 'Mo_1', 'Mo_2']
[7]:
# Check data
pt.plot_cluster(fontsize=8)
2.1.2. Define llineage¶
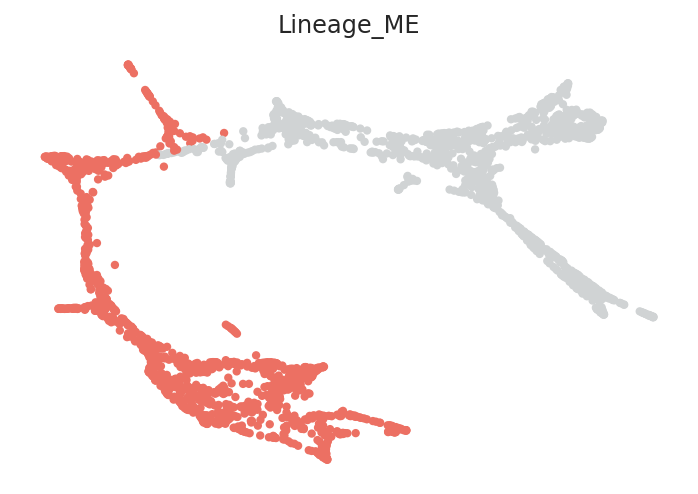
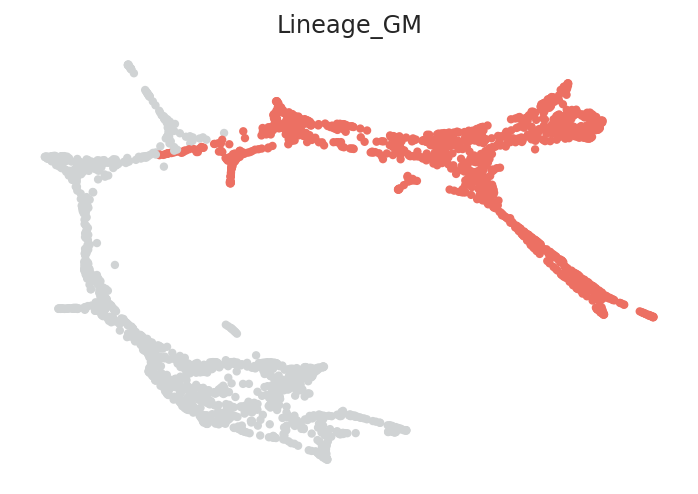
We will make lineage annotation on the scRNA-seq data. For example, the demo scRNA-seq data include roughly two lineages: megakaryocytes-erythroid (ME) lineage and granulocytes-monocyte (GM) lineage.
To get better pseudotime information, calculate the pseudotime for each cell lineage individually. Then, all pseudotime information of each lineage are merged into one.
Lineages can be specified using lists. The lineage structure and number of branches will vary depending on the dataset. Please adjust the code as necessary to match your data.
[8]:
# Here, clusters can be classified into either MEP lineage or GMP lineage
clusters_in_ME_lineage = ['Ery_0', 'Ery_1', 'Ery_2', 'Ery_3', 'Ery_4', 'Ery_5',
'Ery_6', 'Ery_7', 'Ery_8', 'Ery_9', 'MEP_0', 'Mk_0']
clusters_in_GM_lineage = ['GMP_0', 'GMP_1', 'GMP_2', 'GMPl_0', 'GMPl_1', 'Gran_0',
'Gran_1', 'Gran_2', 'Gran_3', 'Mo_0', 'Mo_1', 'Mo_2']
# Make a dictionary
lineage_dictionary = {"Lineage_ME": clusters_in_ME_lineage,
"Lineage_GM": clusters_in_GM_lineage}
# Input lineage information into pseudotime object
pt.set_lineage(lineage_dictionary=lineage_dictionary)
# Visualize lineage information
pt.plot_lineages()
2.2. Add root cell information¶
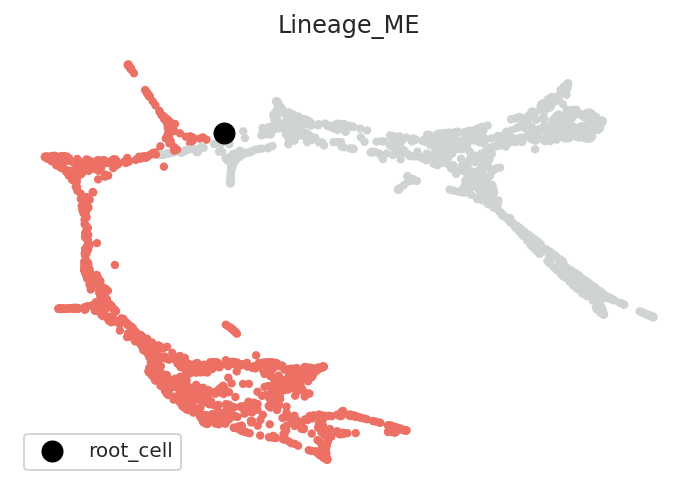
The DPT pseudotime calculation requires the user to specify a root cell. We will manually estimate the root cell for each lineage.
Please read documentation (https://scanpy.readthedocs.io/en/stable/api/scanpy.tl.dpt.html#scanpy.tl.dpt) to find detailed information about the DPT algorithm and root cells
2.2.1. (Optional step) Interactive visualization of cell name¶
We recommend using another python package, plotly to find the cell id for root cell.
Please install plotly in advance.
pip install plotly
Plotly is a toolkit for interactive visualization. We recommend using plotly to pick up root cells in this notebook. For more information, please look at plotly web site. https://plotly.com
Using plotly, we can visualize cell IDs interactively. It helps us pick up a root cell. This is an example image. |00a28c43e0ff4d52bbe8697f2c519730|
https://raw.githubusercontent.com/morris-lab/CellOracle/master/docs/demo_data/screenshot_01.png
[ ]:
# Show interactive plot using plotly. Please make sure that plotly is installed.
try:
import plotly.express as px
def plot(adata, embedding_key, cluster_column_name):
embedding = adata.obsm[embedding_key]
df = pd.DataFrame(embedding, columns=["x", "y"])
df["cluster"] = adata.obs[cluster_column_name].values
df["label"] = adata.obs.index.values
fig = px.scatter(df, x="x", y="y", hover_name=df["label"], color="cluster")
fig.show()
plot(adata=pt.adata,
embedding_key=pt.obsm_key,
cluster_column_name=pt.cluster_column_name)
except:
print("Plotly not found in your environment. Did you install plotly? Please read the instruction above.")
2.2.2. Select root cell for each lineage¶
[10]:
# Estimated root cell name for each lineage
root_cells = {"Lineage_ME": "1539", "Lineage_GM": "2244"}
pt.set_root_cells(root_cells=root_cells)
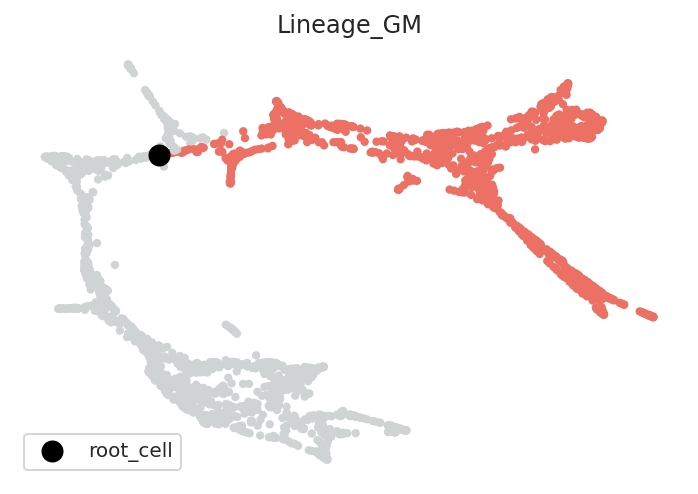
2.2.3. Visualize root cells¶
[11]:
# Check root cell and lineage
pt.plot_root_cells()
2.3. Pseudotime calculation¶
You need to calculate neighbors and the diffusion map before funning DPT. If you have processed the scRNA-seq data according to our tutorial, these calculations have already been performed. - Neighbor calculation: https://scanpy.readthedocs.io/en/stable/generated/scanpy.pp.neighbors.html#scanpy.pp.neighbors - Diffusion map calculation: https://scanpy.readthedocs.io/en/stable/generated/scanpy.tl.diffmap.html#scanpy.tl.diffmap
2.3.1. Check diffusion map¶
[12]:
# Check diffusion map data.
"X_diffmap" in pt.adata.obsm
[12]:
True
Calculate diffusion map if your anndata object does not have diffusion map data.
[13]:
# sc.pp.neighbors(pt.adata, n_neighbors=30)
# sc.tl.diffmap(pt.adata)
2.3.2. Calculate pseudotime¶
[13]:
# Calculate pseudotime
pt.get_pseudotime_per_each_lineage()
# Check results
pt.plot_pseudotime(cmap="rainbow")
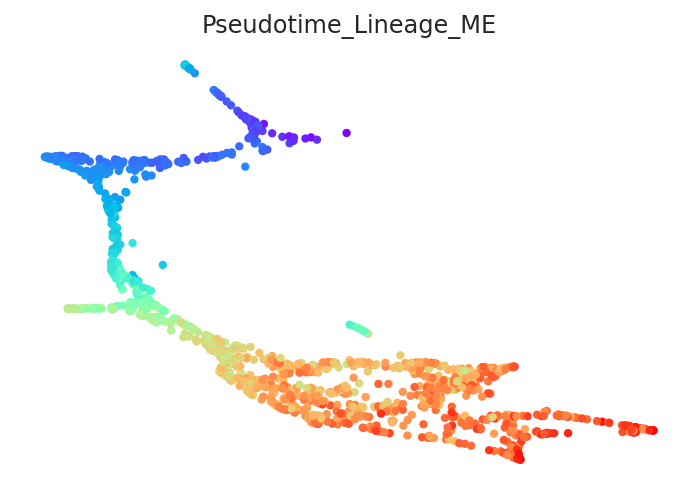
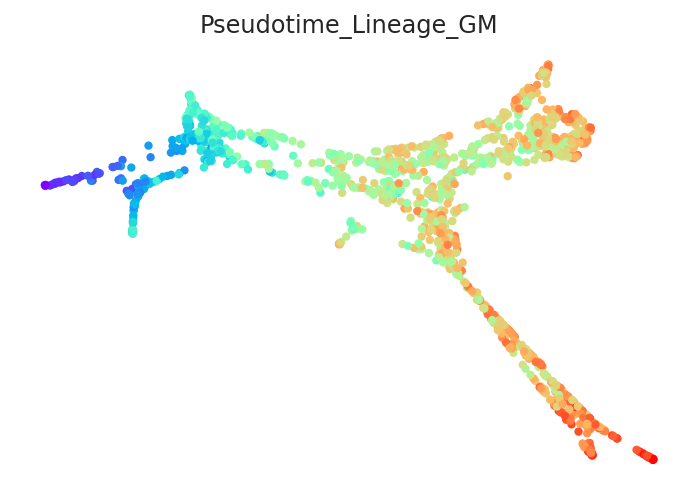
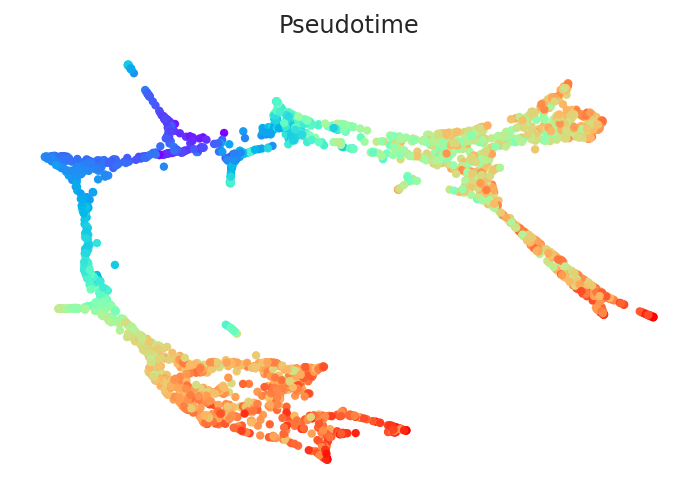
Pseudotime data is stored in the pt.adata.obs.Pseudotime
[14]:
# Check result
pt.adata.obs[["Pseudotime"]].head()
[14]:
| Pseudotime | |
|---|---|
| index | |
| 0 | 0.175565 |
| 1 | 0.712654 |
| 2 | 0.953920 |
| 3 | 0.642302 |
| 4 | 0.951093 |
3. Save data¶
3.1. If you started calculation with an oracle object¶
[15]:
# Add calculated pseudotime data to the oracle object
oracle.adata.obs = pt.adata.obs
# Save updated oracle object
#oracle.to_hdf5(FILE_PATH)
3.2. If you started calculation with anndata¶
[ ]:
# Add calculated pseudotime data to the oracle object
#adata.obs = pt.adata.obs
# Save updated anndata object
#adata.write_h5ad(FILE_PATH)